Anti-Claudin-1 antibodies as a promising
approach in oncology and fibrosis
Novel therapies for cancer and fibrosis
We develop first-in-class anti-Claudin-1 (CLDN1) antibodies for the treatment of CLDN1 positive (CLDN1+) tumors and organ fibrosis. We are rapidly advancing our two lead monoclonal antibodies (mAbs) through clinical development.
Our preclinical programs are next-generation anti-CLDN1 therapies, including antibody-drug conjugates (ADCs), bispecific antibodies, and T-cell engagers.
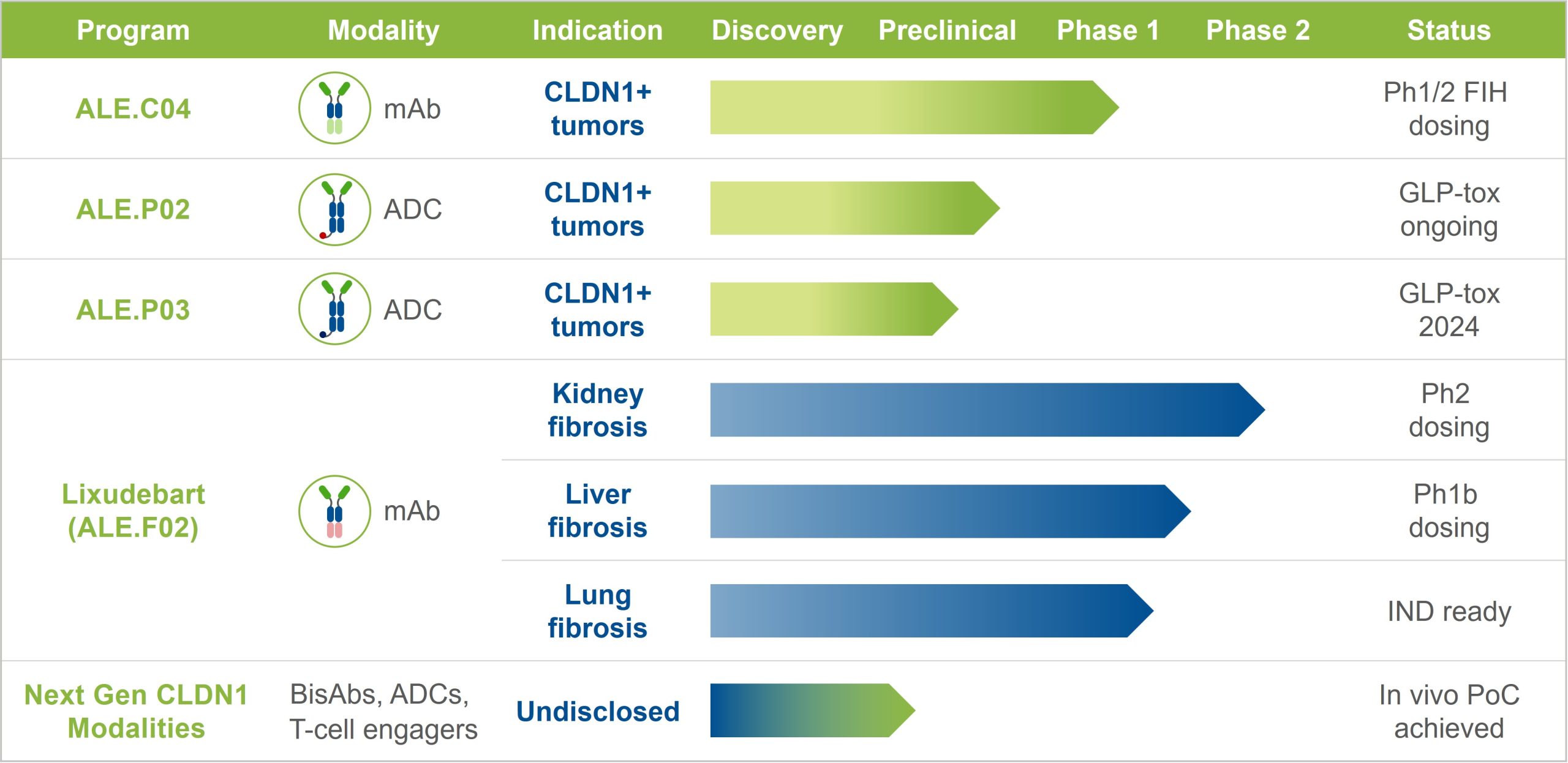
Research and development pipeline
Claudin-1 – a promising therapeutic target
Claudin-1 is a member of the tight junction protein family; its essential role is binding epithelial cells together. In fibrosis and many cancers, Claudin-1 is overexpressed and exposed outside of tight junctions. Here it drives the remodeling of the extracellular matrix, forming a physical barrier that protects tumors from the immune system and causes organ failure in fibrosis. Our founder, Prof. Thomas Baumert discovered an antibody that only binds exposed Claudin-1, unlocking a new promising approach to modify the course of disease.
ALE.C04 – oncology
ALE.C04 is a first-in-class monoclonal antibody developed for CLDN1+ cancer indications. It is designed to selectively kill CLDN1+ tumor cells and open the collagen barrier to enable immunotherapies and the immune system to attack the tumor.
- Phase 1/2 trial in Head and Neck Squamous Cell Carcinoma (NCT06054477) – Recruiting
- FDA Fast Track designation granted
ALE.P02 & ALE.P03 – ADCs for oncology
ALE.P02 and ALE.P03 are first-in-class antibody-drug conjugates (ADCs) in preclinical development for CLDN1+ cancer indications.
- ALE.P02 is undergoing GLP toxicology studies, with a first-in-human clinical trial planned in patients with CLDN1+ tumors.
- For ALE.P03, GLP toxicology studies are being planned.
Lixudebart – fibrosis
Lixudebart (formerly ALE.F02) is a first-in-class antibody developed for liver, kidney and lung fibrosis, in particular for ANCA-associated vasculitis and idiopathic pulmonary fibrosis (IPF). It is designed to reverse fibrosis by blocking fibrotic signaling and opening the collagen barrier to preserve or restore organ function.
- Phase 1 trial in healthy volunteers (SAD and MAD results) – Completed
- Phase 1/2 trial in ANCA-associated vasculitis with renal involvement (NCT06047171) – Recruiting
- Phase 1b trial in advanced liver fibrosis (NCT05939947) – Recruiting
- Phase 2 trial in idiopathic pulmonary fibrosis – Planned
