ALE.P02 & ALE.P03 ADCs for Claudin-1 positive tumors
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) have long been described as the ´magic bullet´ in treating cancer. We have developed ALE.P02 and ALE.P03, two novel ADCs with first-in-class potential in Claudin-1 positive solid tumors. Our ADCs are designed by linking potent cancer drugs to our anti-CLDN1 antibody, creating a powerful new tool to fight cancer.
The role of Claudin-1 in solid tumors
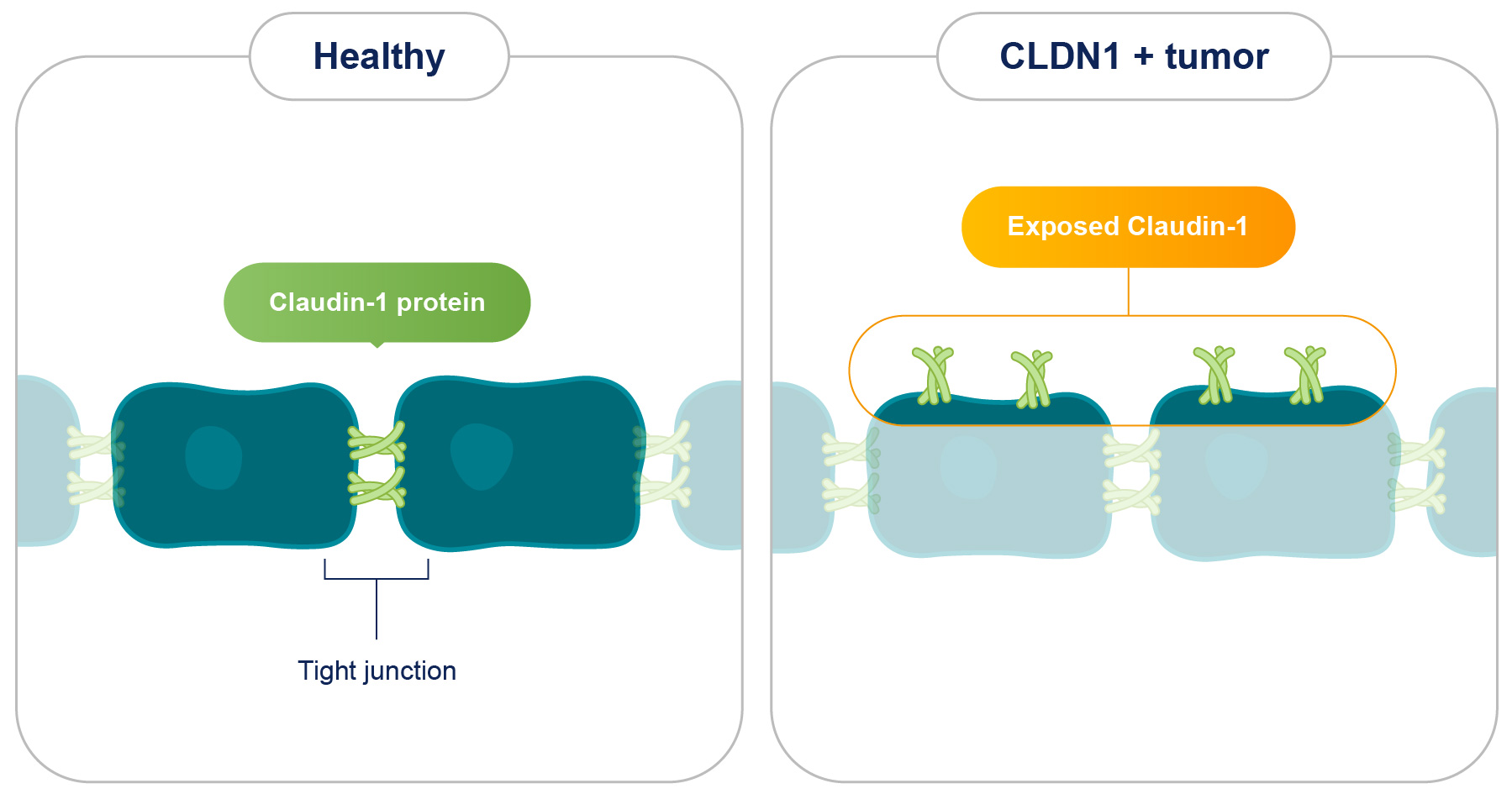
In healthy cells, Claudin-1 is hidden within tight junctions where its normal function is to bind cells together. In many solid tumors, Claudin-1 (CLDN1) is overexpressed and exposed outside tight junctions thus providing a unique “tumor-tag” that is selectively recognized by our anti-CLDN1 antibody without impacting healthy tissues.
What is an ADC
Antibody-drug conjugates (ADCs) are an innovative class of therapy where a payload (drug) is coupled to a monoclonal antibody using a linker. Nowadays, ADCs are a widely used tool for the management or cure of cancer.
Safer and more effective cancer treatments
Chemotherapy and other cancer drugs often have low specificity towards tumor cells, causing poor therapeutic responses and significant toxicity to normal healthy tissues. ADCs can selectively target and kill tumor cells decreasing toxicity without restricting anti-cancer activity.
Claudin-1: a promising new target for ADCs
At Alentis we developed an antibody that specifically binds CLDN1+ tumor cells. By arming this antibody with two differentiated cancer drugs (payloads) we engineered our novel anti-CLDN1 ADCs; ALE.P02 (tubulin inhibitor ADC) and ALE.P03 (topoisomerase I inhibitor ADC).
Upon binding CLDN1+ tumor cells, the ADCs are internalized, delivering their potent cancer drugs selectively and directly into the tumor tissue, while sparing healthy tissue.
Anti-CLDN1 antibody-drug conjugates (ADCs)
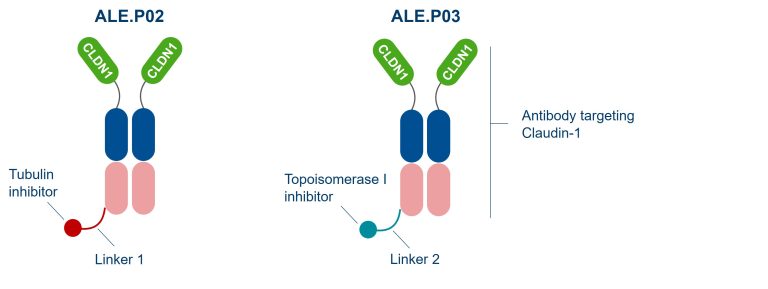
ADC designs for ALE.P02 and ALE.P03 including an anti-CLDN1 antibody, linker and payloads (tubulin inhibitor or topoisomerase I inhibitor).
Clinically validated linker and payloads
The linkers and payloads used in designing ALE.P02 and ALE.P03 are clinically validated. Combining our novel anti-CLDN1 antibody with these validated components potentially de-risk the clinical development of our ADCs.
Clinical trials
ALE.P02 Phase 1/2 clinical trial - ongoing
ALE.P02 is being studied in a first-in-human clinical trial (NCT06747585). The study is planned to enroll 170 patients with advanced or metastatic CLDN1+ squamous solid tumors including but not limited to lung, head and neck, cervical and esophageal cancers.
The FDA granted ALE.P02 Fast Track designation for the treatment of advanced or metastatic CLDN1+ squamous cancers irrespective of the organ of origin.
ALE.P03 Phase 1/2 clinical trial - ongoing
For ALE.P03, a first-in-human clinical trial (NCT07169734) is ongoing in patients with CLDN1+ solid tumors.
Preclinical evidence
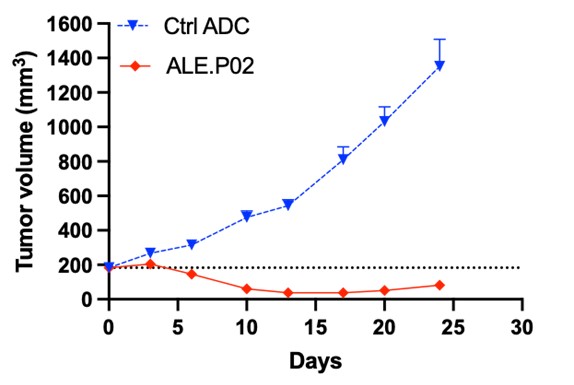
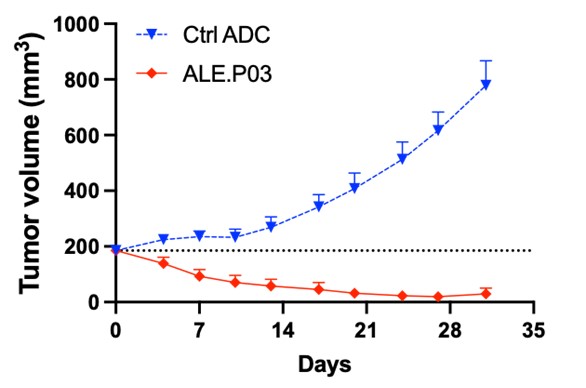
Preclinical studies of ALE.P02 and ALE.P03 confirmed selective binding and internalization in CLDN1+ tumor cells leading to strong tumor growth inhibition across both different tumor models and CLDN1 expression levels in vivo.
A single dose of ALE.P02 or ALE.P03 resulted in complete tumor regression in Patient-Derived-Xenograft in vivo models